Membres de l'équipe
Thomas Rival
Plasticité des cellules souches
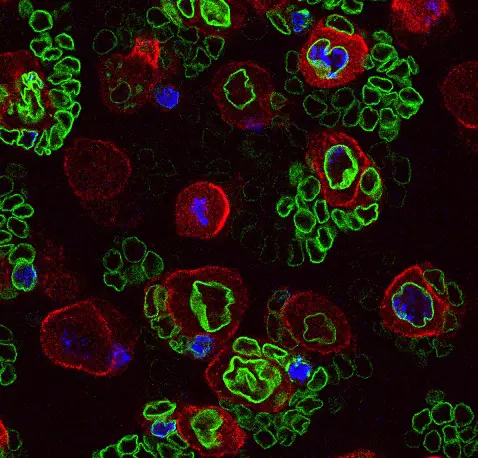
Notre équipe décrypte les mécanismes qui contrôlent la prolifération des cellules souches au cours du développement, des cancers de l'enfant et de la régénération des tissus. Nous étudions principalement ces processus dans le système nerveux central.
Lorsque nous comprendrons parfaitement comment les cellules souches sont contrôlées dans notre corps, pourrons-nous régénérer les organes blessés ou guérir les cancers ?
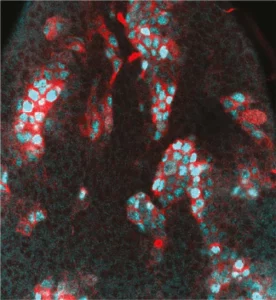
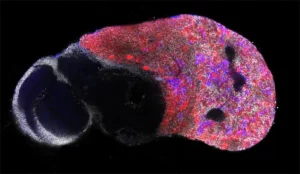
Notre laboratoire combine deux des organismes modèles les plus puissants (la drosophile et l’embryon aviaire) avec de nouvelles technologies pour manipuler et étudier les cellules souches neurales avec une précision moléculaire sans précédent. Cela nous permet de déchiffrer les différents niveaux de régulation contrôlant le déroulement des programmes génétiques dans les cellules souches au cours du développement normal, de la régénération et dans les cancers ayant des origines développementales comme les cancers pédiatriques.
Nous étudions comment les cellules souches sont contrôlées par l’épigénétique, la transcription et la traduction, mais aussi par les signaux biochimiques et mécaniques produits par leur environnement.
Rejoignez-nous si vous voulez aussi contribuer!
Publications
Regulation of developmental hierarchy in Drosophila neural stem cell tumors by COMPASS and Polycomb complexes
Coopted temporal patterning governs cellular hierarchy, heterogeneity and metabolism in Drosophila neuroblast tumors
Developmental regulation of regenerative potential in Drosophila by ecdysone through a bistable loop of ZBTB transcription factors
Neural stem cell-encoded temporal patterning delineates an early window of malignant susceptibility in Drosophila
Protection of neuronal diversity at the expense of neuronal numbers during nutrient restriction in the Drosophila visual system
Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila
In vivo AGO-APP identifies a module of microRNAs cooperatively preserving neural progenitors
In vivo AGO-APP identifies a module of microRNAs cooperatively preserving neural progenitors
Serotonin signaling regulates actomyosin contractility during morphogenesis in evolutionarily divergent lineages
Regulation of developmental hierarchy in Drosophila neural stem cell tumors by COMPASS and Polycomb complexes
Temporal patterning in neural progenitors: from Drosophila development to childhood cancers
Coopted temporal patterning governs cellular hierarchy, heterogeneity and metabolism in Drosophila neuroblast tumors
Developmental regulation of regenerative potential in Drosophila by ecdysone through a bistable loop of ZBTB transcription factors
Two distinct mechanisms silence chinmo in Drosophila neuroblasts and neuroepithelial cells to limit their self-renewal
Neural stem cell-encoded temporal patterning delineates an early window of malignant susceptibility in Drosophila
Building a brain under nutritional restriction: insights on sparing and plasticity from Drosophila studies.
Protection of neuronal diversity at the expense of neuronal numbers during nutrient restriction in the Drosophila visual system
Temporal specification of neural stem cells: insights from Drosophila neuroblasts.
Temporal control of neuronal diversity: common regulatory principles in insects and vertebrates?
Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila
Signaling meets chromatin during tissue regeneration in Drosophila.
Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs.
Brainy but not too brainy: starting and stopping neuroblast divisions in Drosophila.
Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound.
A cellular memory module conveys epigenetic inheritance of hedgehog expression during Drosophila wing imaginal disc development.
Actualités
Fais ton stage à l’IBDM !
Tu es à la recherche de ton stage de Master ? L’IBDM te semble être le bon endroit pour le faire ? Découvre nos offres dès maintenant.
Jeunesse & Science : Delta Festival
Delta Festival 2023 : Musique, villages à thèmes, et une touche d’IBDM pour briller sous le soleil de Marseille.
Félicitation à Robert Kelly, Frank Schnorrer, Cédric Maurange, Bianca Habermann et Delphine Delacour !
L’IBDM inspire les jeunes esprits : en impliquant les enfants des écoles primaires dans la lutte contre le cancer pédiatrique (“Contre le cancer, j’apporte ma pierre”) et en interagissant avec les lycéens grâce à des expériences immersives (DECLICS).
Epigénétique et hiérarchie tumorale
7 équipes de l’IBDM ont reçu une bourse ANR
7 équipes de l’IBDM ont reçu des subventions de l’Agence Nationale pour la Recherche (ANR) en 2021. Félicitations à Vincent Bertrand, Harold Cremer, Pascale Durbec,
Les mécanismes des cancers du cerveau avec une origine développementale révélés par la drosophile
Dans un travail récent publié dans eLife, l’équipe de Cédric Maurange identifie un programme génétique organisant l’hétérogénéité métabolique des cellules dans un modèle de tumeur neurale pédiatrique.
During this M2 project, the student will work on a recently developed model of medulloblastoma in the chick embryo.
We welcome applications from highly motivated, curiosity-driven PhD candidates, interested in the topic of Developmental Neurobiology, to join Cédric Maurange’s team. The position is funded for 3 years. Research in