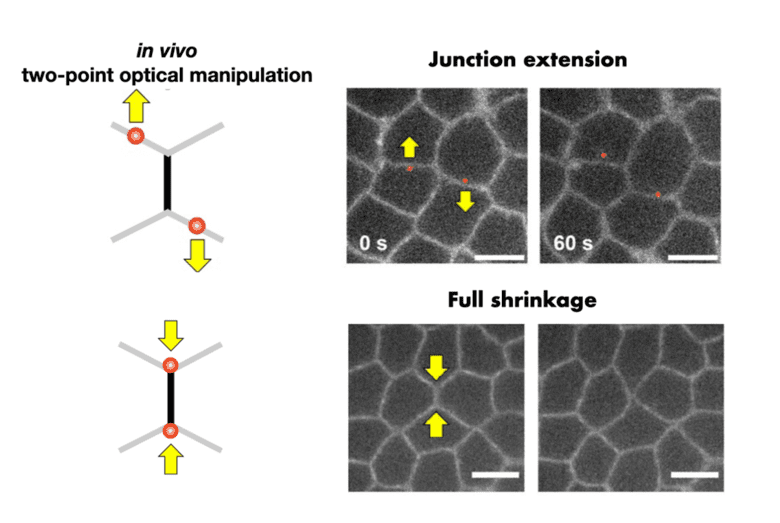
Under too much stretch, a rubber band yields and undergoes irreversible deformation. To which extent does a cell-cell junction behave similarly under mechanical stress? This question is of interest to understanding embryogenesis, where the cell-based living material undergoes a series of irreversible deformation.
In a recently accepted work, Kenji Nishizawa, Claire Chardès, and Pierre-François Lenne designed an optical system to stretch cell-cell junction in vivo during early Drosophila embryogenesis. The idea is to split a laser beam into two beams, moved separately to produce two independent traps. These two optical traps are then used to put cell-cell junctions under tension or compression. The method is non-invasive in that forces are exerted directly on the cell endogenous material, unlike alternative methods, which rely on injecting beads. The Lenne team turned to theoreticians Shao-Zhen Lin & Jean-François Rupprecht at CPT, who adapted a mechanical model of cellular assemblies, called the vertex-based models. The model showed that the measured cell-cell junction length could only be explained through a combination of irreversible, viscous elongation and Myosin-II activation when the junction was put under compression. This research sheds light on how individual cells within epithelial tissue deform and respond to forces.
Figure legend
Two-point optical manipulation of cell-cell contacts in the early epithelium of Drosophila embryo
To know more
Nishizawa K, Lin SZ, Chardès C, Rupprecht JF, Lenne PF. Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo. Proc Natl Acad Sci U S A. 2023 Mar 28;120(13):e2212389120. doi: 10.1073/pnas.2212389120. Epub 2023 Mar 22. PMID: 36947511.