Epithelial monolayer morphogenesis – E2M
Our aim is to reveal the principles underlying the organization of a monolayered epithelium to ensure functionality and homeostasis.
Epithelia act as a physical barrier against external aggressions but also ensure organ functionality. If not correctly assembled or dysfunctional, this leads to pathological situations that include a variety of diseases spanning from rare developmental syndromes or cancer.
How epithelia coordinate and harmonize the responses of each cell with not only their nearest neighbors, but entire tissue, to guarantee proper spatial arrangement, integrity and functionality is still not well understood. So far, the question of epithelial coherence has been mainly tackled in invertebrate models during development or in transformed cell lines in culture.
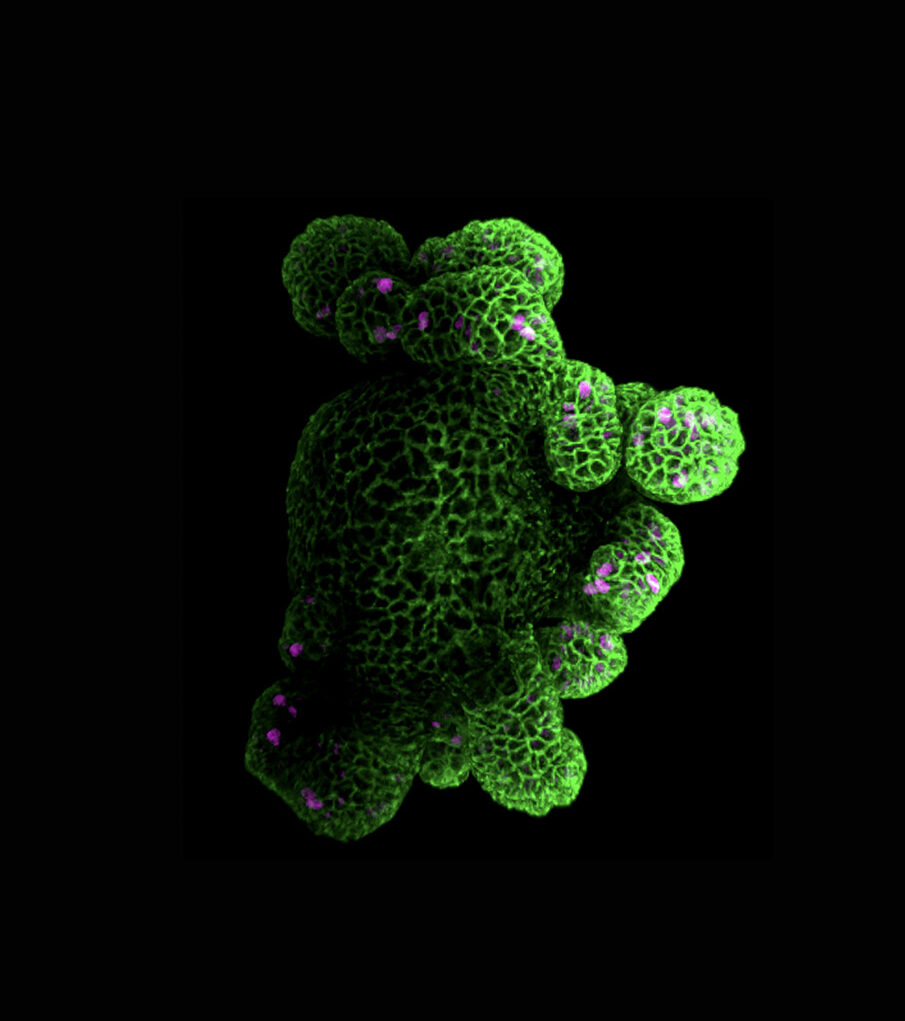
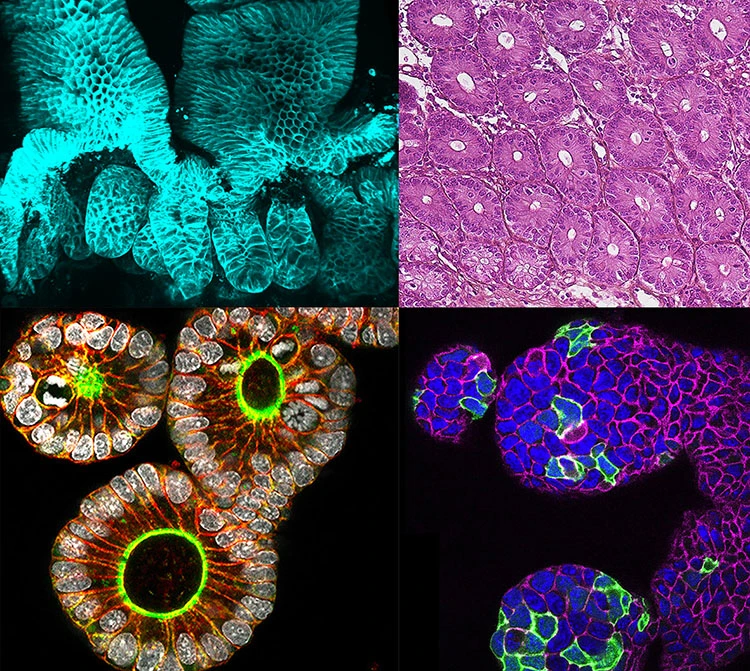
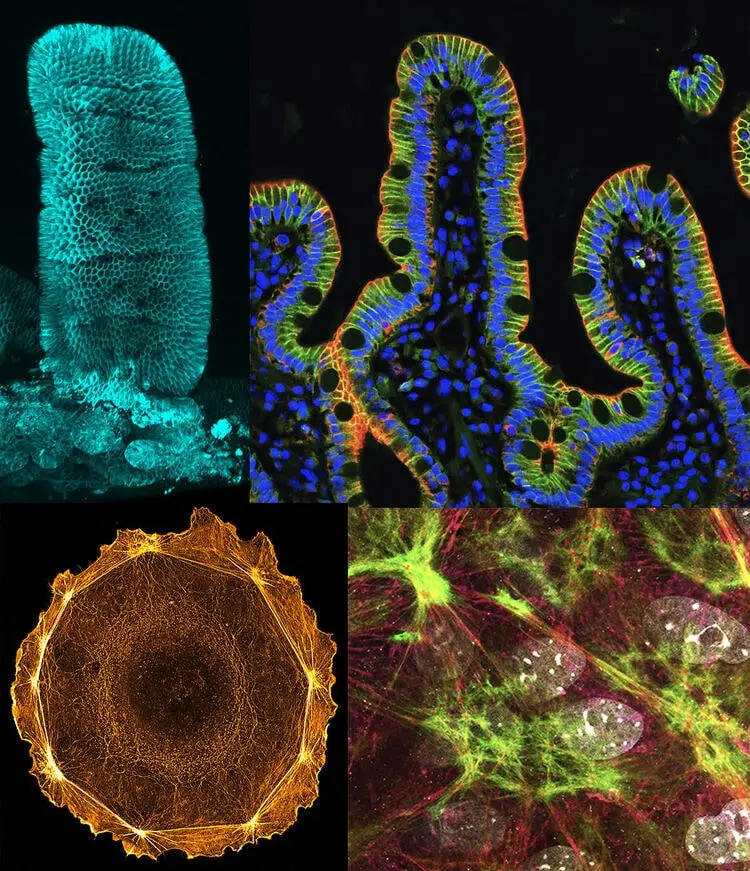
The intestinal epithelium is a tremendous model for this field of research, as it is one of the most fast-proliferative and regenerative in mammalian organisms. In addition, the intestinal tissue is subject to adverse situations, where the balance between cell proliferation, differentiation and death has to be strictly maintained. However, the study of the cellular and developmental mechanisms that govern the development and architecture of the intestinal tissue is still in its infancy.
The general objective of our project focuses on understanding the determination and maintenance of intestinal functional domains, and to evaluate their spatiotemporal coordination.
More specifically, our aims are:
- to define mechanisms controlling the integrity of the proliferative domain, and assess their impact on the crypt development;
- to characterize the epithelial connectivity and collective behavior of the differentiated domain in homeostatic conditions or under challenging contexts.
One of the strong aspects of our research is to confront in vivo and in vitro murine and human disease models, and to combine different approaches from advanced cell biology, tissue engineering, histology, molecular biology, biophysics and computational modelling. This strategy allows us to determine the adaptive processes that epithelial cells employ to polarize and organize in a given environment, and enhance their ability to modulate their fate.
Publications
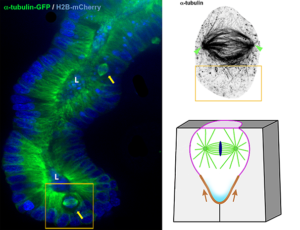
Length-limitation of astral microtubules orients cell divisions in intestinal crypts
Regulation and functions of cell division in the intestinal tissue
Modulation of designer biomimetic matrices for optimized differentiated intestinal epithelial cultures
V0-ATPase downregulation induces MVID-like brush border defects independently of apical trafficking in the mammalian intestine.
A role of astral microtubules in the orientation of cell division: when length counts…too!
A multicellular actin star network underpins epithelial organization and connectivity
Length-limitation of astral microtubules orients cell divisions in intestinal crypts
Regulation and functions of cell division in the intestinal tissue
The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces_edited
Modulation of designer biomimetic matrices for optimized differentiated intestinal epithelial cultures
News
Join the IBDM for your internship!
Seeking for your Master internship? The IBDM seems like the right place to do it? Check out our offers.
Congratulations to Robert Kelly, Frank Schnorrer, Cédric Maurange, Bianca Habermann and Delphine Delacour!
IBDM welcomes a new research group!
We are delighted to welcome Delphine Delacour and her new research group ‘Epithelial monolayer morphogenesis’ to IBDM.
A new model for cell division orientation
The Delacour group proposes a new mechanism for the control of planar spindle orientation and monolayered tissue architecture in the intestinal epithelium.
The master project (M2) aims at characterizing the epithelial connectivity and collective behavior of the intestinal differentiated domain in homeostatic conditions or under challenging contexts.