Genetic control of heart development
Our team studies heart development in order to identify biological mechanisms underlying organogenesis, regeneration and congenital disease.
Learning how organs form in the embryo is essential to understand the origins of congenital disease and to develop approaches for repairing adult tissue after damage. The heart is the first organ to form and function in the embryo and cardiac development involves complex interactions between genes, progenitor cell populations and inter-cellular signaling events. This complexity is reflected in the fact that congenital heart defects affect 1 in 100 births. Our group studies heart development in the mouse, where the developmental sequence of events is very similar to that in humans, focusing on two critical areas.
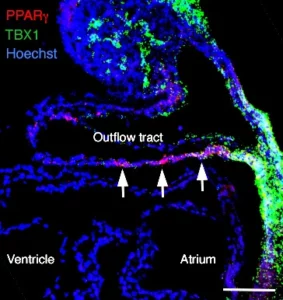
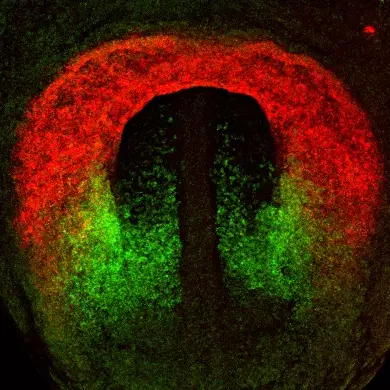
Firstly, we investigate the growth of the embryonic heart by progressive addition of myocardium from progenitor cells known as the second heart field (SHF). SHF derived parts of the heart are hotspots for common congenital heart defects. We study the properties of SHF cells and the mechanisms driving their deployment to the heart. The genetic program of the SHF is shared with head muscle progenitor cells, and we also investigate how a common program diverges to give rise to heart and head muscle.
Secondly, we study the development of the cardiac conduction system that forms the electrical wiring of the heart and coordinates the heartbeat. The conduction system is derived from common progenitor cells with contractile cardiomyoctyes of the heart and we investigate the cellular and genetic mechanisms required for the establishment of these specialized myocytes during normal development and under pathological conditions.
Publications
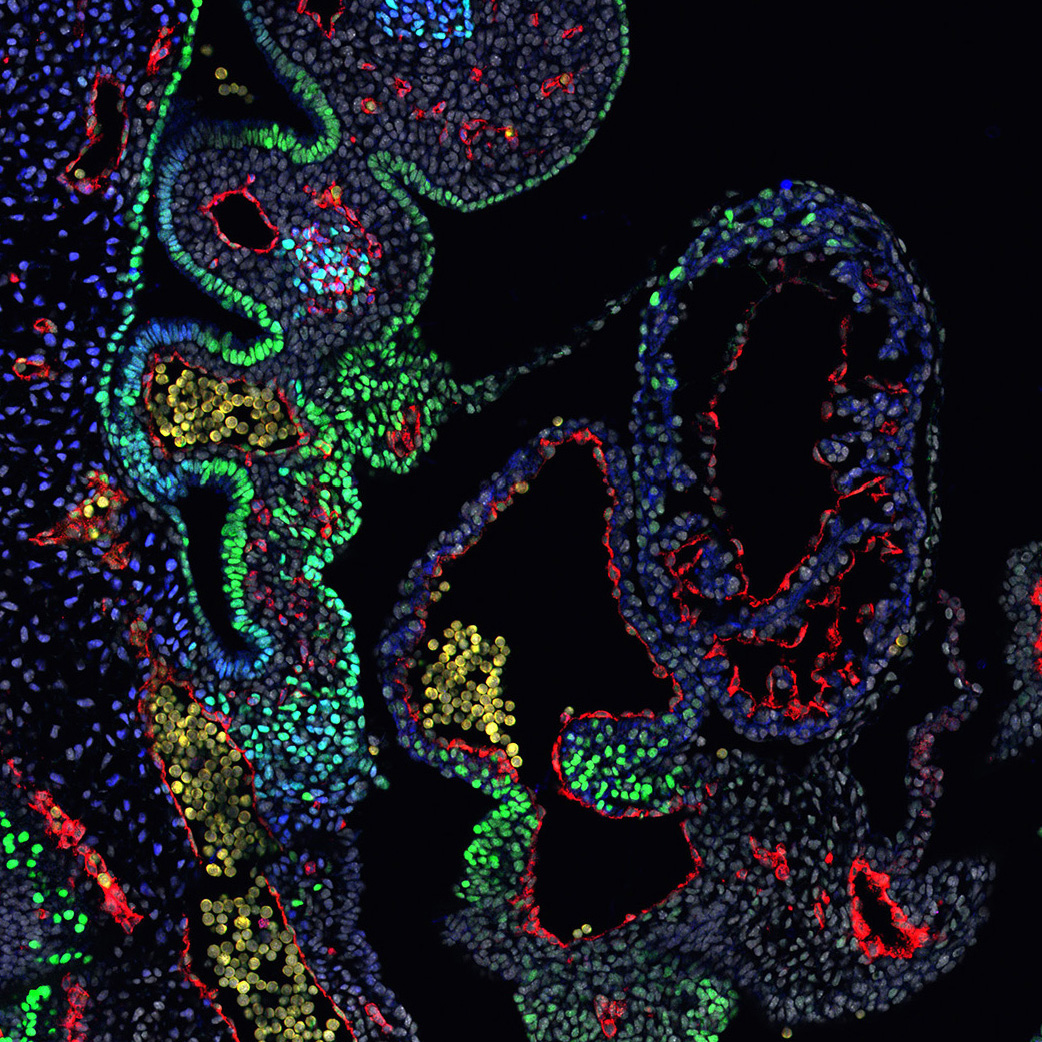
Nkx2-5 defines distinct scaffold and recruitment phases during formation of the murine cardiac Purkinje fiber network
Cardiopharyngeal mesoderm origins of musculoskeletal and connective tissues in the mammalian pharynx
T-box genes and retinoic acid signaling regulate the segregation of arterial and venous pole progenitor cells in the murine second heart field
Epithelial tension in the second heart field promotes mouse heart tube elongation.
Tbx1 Coordinates Addition of Posterior Second Heart Field Progenitor Cells to the Arterial and Venous Poles of the Heart
Single-cell morphometrics reveals T-box gene-dependent patterns of epithelial tension in the Second Heart field
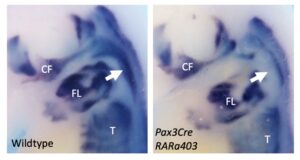
Retinoic acid signalling regulates branchiomeric neck muscle development at the head/trunk interface
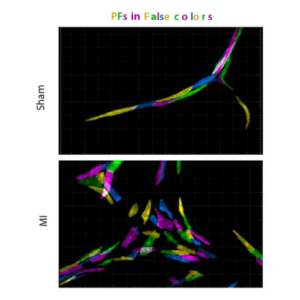
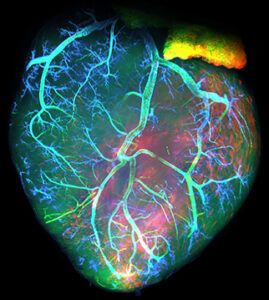
Transient formation of collaterals contributes to the restoration of the arterial tree during cardiac regeneration in neonatal mice
Nkx2-5 loss of function in the his-purkinje system hampers its maturation and leads to mechanical dysfunction
Capturing Cardiogenesis in Gastruloids
Nkx2-5 defines distinct scaffold and recruitment phases during formation of the murine cardiac Purkinje fiber network
Hox-dependent coordination of mouse cardiac progenitor cell patterning and differentiation
Cardiopharyngeal mesoderm origins of musculoskeletal and connective tissues in the mammalian pharynx
Straightjacket/α2δ3 deregulation is associated with cardiac conduction defects in myotonic dystrophy type 1
Defects in Trabecular Development Contribute to Left Ventricular Noncompaction
Loss of Tbx3 in murine neural crest reduces enteric glia and causes cleft palate, but does not influence heart development or bowel transit
Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues
T-box genes and retinoic acid signaling regulate the segregation of arterial and venous pole progenitor cells in the murine second heart field
Embryonic Tbx3 + cardiomyocytes form the mature cardiac conduction system by progressive fate restriction
Epithelial Properties of the Second Heart Field
Epithelial tension in the second heart field promotes mouse heart tube elongation.
Coronary stem development in wildtype and Tbx1 null mouse hearts.
Revascularization of the heart after infarct: lessons from embryonic development.
A Cranial Mesoderm Origin for Esophagus Striated Muscles.
Adhesive Enrichment and Membrane Turnover at the Heart of Cardiopharyngeal Induction.
Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of ChannelRhodopsin-2.
Endothelial Plasticity Drives Arterial Remodeling Within the Endocardium After Myocardial Infarction
A new heart for a new head in vertebrate cardiopharyngeal evolution.
Loss of Wnt5a disrupts second heart field cell deployment and may contribute to OFT malformations in DiGeorge syndrome.
Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium.
FGF10 promotes regional foetal cardiomyocyte proliferation and adult cardiomyocyte cell-cycle re-entry.
TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field.
Tbx1 Coordinates Addition of Posterior Second Heart Field Progenitor Cells to the Arterial and Venous Poles of the Heart
Prdm1 functions in the mesoderm of the second heart field, where it interacts genetically with Tbx1, during outflow tract morphogenesis in the mouse embryo
Cardiac arrhythmia induced by genetic silencing of ‘funny’ (f) channels is rescued by GIRK4 inactivation.
Resolving cell lineage contributions to the ventricular conduction system with a Cx40-GFP allele: a dual contribution of the first and second heart fields.
Second heart field cardiac progenitor cells in the early mouse embryo.
Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium.
The effect of connexin40 deficiency on ventricular conduction system function during development.
Epistatic rescue of Nkx2.5 adult cardiac conduction disease phenotypes by prospero-related homeobox protein 1 and HDAC3.
Remodeling of the peripheral cardiac conduction system in response to pressure overload.
Identification of a Tbx1/Tbx2/Tbx3 genetic pathway governing pharyngeal and arterial pole morphogenesis.
Tbx1, subpulmonary myocardium and conotruncal congenital heart defects.
Inducible Cx40-Cre expression in the cardiac conduction system and arterial endothelial cells.
Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo.
Biphasic development of the mammalian ventricular conduction system.
Hes1 expression is reduced in Tbx1 null cells and is required for the development of structures affected in 22q11 deletion syndrome.
Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of DiGeorge syndrome.
Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries.
Megavoltage planar and cone-beam imaging with low-Z targets: dependence of image quality improvement on beam energy and patient separation.
Hes1 is expressed in the second heart field and is required for outflow tract development.
Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates.
Signaling pathways controlling second heart field development.
Relationship between neural crest cells and cranial mesoderm during head muscle development.
Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos.
The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning.
Integration of embryonic and fetal skeletal myogenic programs at the myosin light chain 1f/3f locus.
Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field.
Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene.
Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice.
Left and right ventricular contributions to the formation of the interventricular septum in the mouse heart.
Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries.
The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis.
Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development.
The clonal origin of myocardial cells in different regions of the embryonic mouse heart.
Cell history determines the maintenance of transcriptional differences between left and right ventricular cardiomyocytes in the developing mouse heart.
The anterior heart-forming field: voyage to the arterial pole of the heart.
The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm.
News
Muscle formation at the head/trunk interface
Trapezius muscle development requires crosstalk between pharyngeal and somitic mesoderm in the early mouse embryo.
Cardiac regeneration leads to hyperplastic Purkinje fiber network in association with ventricular conduction disorders.
Repair of cardiac coronary using collaterals
During cardiac regeneration, collaterals form rapidly to perfuse the infarcted area and help repair the coronary artery.
Congratulations to Robert Kelly, Frank Schnorrer, Cédric Maurange, Bianca Habermann and Delphine Delacour!
A publication from the Kelly team in Nature Communications, conducted by Caroline Choquet and Lucile Miquerol, focuses on the development of cardiac Purkinje fibers that serve as electrical cables in the ventricles to synchronize heartbeats.
Cardiopharyngeal mesoderm origins of musculosketelal and connective tissues in the mammalian pharynx
In a study published in Development, Adachi et al used genetic lineage tracing to investigate the cellular origin of the throat in the mouse model.
No jobs opportunities found..