Maina Team publishes in Advanced Science
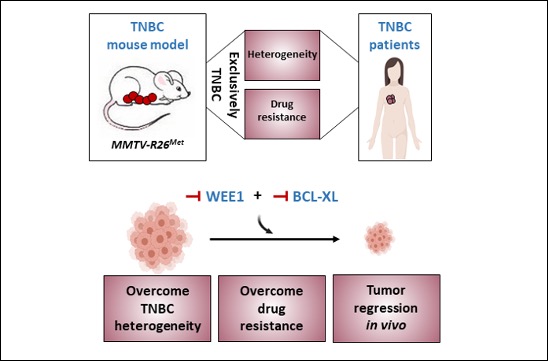
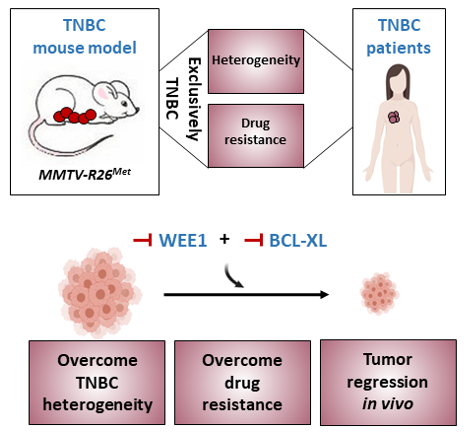
Maina’s team has generated a genetic system that models, in mice, the heterogeneity and primary resistance to treatment of human Triple Negative Breast Cancer (TNBC), a highly aggressive type of breast cancer for which there is currently no effective treatment. In an international collaborative setting, they also discovered a treatment that overcomes TNBC heterogeneity and resistance. Published in the journal Advanced Science, this work shows how potentially relevant treatments can be identified by modelling the disease.
Modelling Heterogeneity of Triple-Negative Breast Cancer Uncovers a Novel Combinatorial Treatment Overcoming Primary Drug Resistance
Breast cancer is a global health problem. Several laboratories are exploring different strategies to identify early detection methods and targeted therapies. Among the four major classes of breast cancer, Triple-Negative Breast Cancer (TNBC), which accounts for 10-15% of all breast cancers, has a poor prognosis and is the most complex to control. Radiotherapy and chemotherapy are currently the main treatments for TNBC, although only effective in few patients. At present, knowledge about TNBC and the identification of effective therapies is limited by the lack of relevant preclinical models capable of recapitulating the complexity of TNBC, in particular the extraordinary heterogeneity between tumours and the primary resistance to treatment. It is therefore crucial to better understand this pathology.
The mouse model generated by Maina’s team is based on a slight perturbation of signals involved in the regulation of mammary gland function. Although this perturbation is controlled by the tissue, over time it leads to alterations that induce spontaneous tumour formation in mice. An unexpected aspect is the specificity of the tumours generated, which are exclusively of the TNBC type. Nevertheless, it is probably the subtle nature of this perturbation that allows the generation of heterogeneous molecular alterations among different mouse tumours, as observed in TNBC patients. In addition, this mouse model summarizes the resistance to conventional chemotherapy, frequently observed in patients. All of these characteristics demonstrate the relevance of this mouse model for the study of the mechanisms underlying TNBC. Through collaborative studies, at the national and international level, the researchers have employed this model to identify a combination of blocking agents capable of killing murine and human TNBC cells very efficiently, overcoming heterogeneity and primary resistance.
This research, based on an interdisciplinary strategy, includes mouse genetics, large-scale screening, data interpretation using machine learning, integration of human cancer data, and screening of anti-cancer agents. This work illustrates how a model of human pathology, summarizing its complexity, allows the understanding of the underlying molecular aspects and proposes new treatments to be further explored as potential therapies.
To know more :
Modeling Heterogeneity of Triple‐Negative Breast Cancer Uncovers a Novel Combinatorial Treatment Overcoming Primary Drug Resistance
Fabienne Lamballea,*, Fahmida Ahmada, Yaron Vinik, Olivier Castellanet, Fabrice Daian, Anna-Katharina Müller, Ulrike A. Köhler, Anne-Laure Bailly, Emmanuelle Josselin, Rémy Castellano, Christelle Cayrou, Emmanuelle Charafe-Jauffret, Gordon B. Mills, Vincent Géli, Jean-Paul Borg, Sima Levb, Flavio Mainab,*
Advanced Science: https://doi.org/10.1002/advs.202003049
Contact
Fabienne Lamballe – fabienne.lamballe@univ-amu.fr
Flavio Maina – flavio.maina@univ-amu.fr