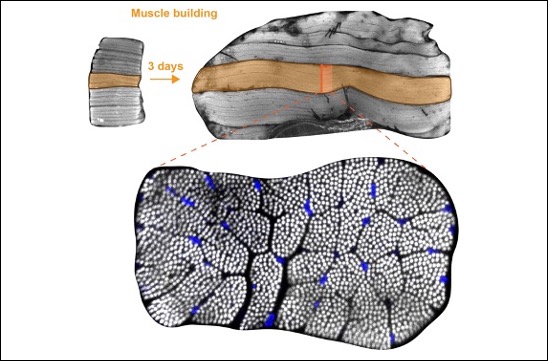
Muscles power animals running, swimming or flying. The forces are produced by chains of actin and myosin motors called myofibrils. During animal development muscles form by fusion of small myoblasts to initially rather small myotubes. These myotubes then build their contractile myofibrils and grow dramatically in size to power future animal movements. How this enormous gain of muscle volume is controlled during development has now been revealed using the fruit fly Drosophila. An interdisciplinary team led by Frank Schnorrer and Bianca Habermann at the Developmental Biology Institute of CNRS & Aix Marseille University, together with Matthias Mann at the Max Planck Institute of Biochemistry in Munich and Bart Deplancke at École Polytechnique Fédérale in Lausanne discovered that a signalling pathway, called the Hippo pathway, is controlling muscle growth during development of Drosophila flight muscles. By combining genetics with proteomics and next generation mRNA sequencing the team found that the Hippo pathway is supervising the production of the contractile proteins actin, myosin and many others that build the contractile myofibrils. Hence, making more of these proteins results in more myofibrils, which directly triggers muscle growth and powerful muscle formation. Similar principles may apply during growth of human muscle, in particular human heart muscle that grows tremendously without the addition of new cells. These new results have just been published in eLife.
The Hippo pathway is a famous conserved gate keeper for tissue growth, discovered in the fruit fly almost 20 years ago. It controls growth also in mammals and is particularly important in cancer development when de-regulated. Most of what we know to date about how the Hippo pathway is regulated stems from studies in epithelial cells, like skin or gut cells. During normal tissue growth the Hippo kinase is inactive, allowing the epithelial cells to grow and divide to fill the available space, matching the growth of the organism. When the available space is filled, tissue mechanics feedbacks and triggers the activation of the Hippo kinase, which stops further growth.
This new study identified an interesting twist how the Hippo pathway is regulated in flight muscle to supervise its growth. In contrast to epithelia, this cell type does not divide anymore but grows by increasing the size of the individual cells, very similar to how human heart is growing during development or exercise. Aynur Kaya-Çopur, first author of the study, found that the Hippo kinase in muscle is regulated by a particular phosphatase complex, called the STRIPAK complex, located at muscle membranes, which are at close contact to the contractile myofibrils. Hence, the authors suggest that a need for growth is sensed mechanically: too small muscles are ‘overstretched’ and hence sense the need to grow. They do so not by cell division but rather by production of more myofibril proteins, which results in more as well as longer and thicker myofibrils. Thus, muscle volume increases. The authors found that Hippo needs to directly bind to the STRIPAK complex in muscle for its normal regulation. How precisely the ‘mechanical signal’ talks to the STRIPAK complex to block Hippo kinase activity remains to be discovered. In the future, it will be exciting to explore if a similar mechanism controls timing and amount of myofibril protein production in the human heart, which also grows tremendously during development or exercise.
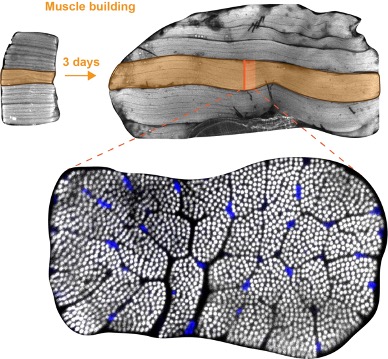
Bottom: cross-section of an individual flight muscle fiber. More than 1000 contractile myofibrils are visible as white dots and nuclei are stained in blue.
To know more :
eLife 2021;10:e63726 DOI: 10.7554/eLife.63726
Aynur Kaya-Çopur Is a corresponding author, Fabio Marchiano, Marco Y Hein, Daniel Alpern, Julie Russeil, Nuno Miguel Luis, Matthias Mann, Bart Deplancke, Bianca H Habermann, Frank Schnorrer
Contact
Frank Schnorrer – frank.SCHNORRER@univ-amu.fr