The team of Alphée Michelot, in collaboration with the team of Pekka Lappalainen (University of Helsinki) identified and characterized a family of highly sensitive fluorescent nucleotide analogues structurally compatible with actin.
Actin dynamics provide force for vital processes of the eukaryotic cell, such as migration or endocytosis. Such dynamics relies on the use of ATP, which binds to actin and provides free energy to sustain these chemical reactions.
However, the understanding of actin dynamics and energetics remains limited due to the lack of high-quality probes. Existing probes are not ideal, because they are not sensitive and cannot be used with fluorescence microscopy.
In this work, the team found that among various chemistries available commercially, N6-(6-Amino)hexyl-ATP derivatives bind to actin with similar characteristics than ATP. These new probes enable monitoring actin assembly and nucleotide exchange with single-molecule microscopy and time-resolved fluorescence anisotropy, therefore providing robust and highly versatile tools to study actin dynamics. In addition to that, these fluorescent nucleotides maintain functional interactions with many actin-binding proteins, are hydrolyzed within actin filaments, and provide energy to power actin-based processes.
These probes represent therefore robust and highly versatile tools to study actin dynamics and functions of actin-binding proteins.
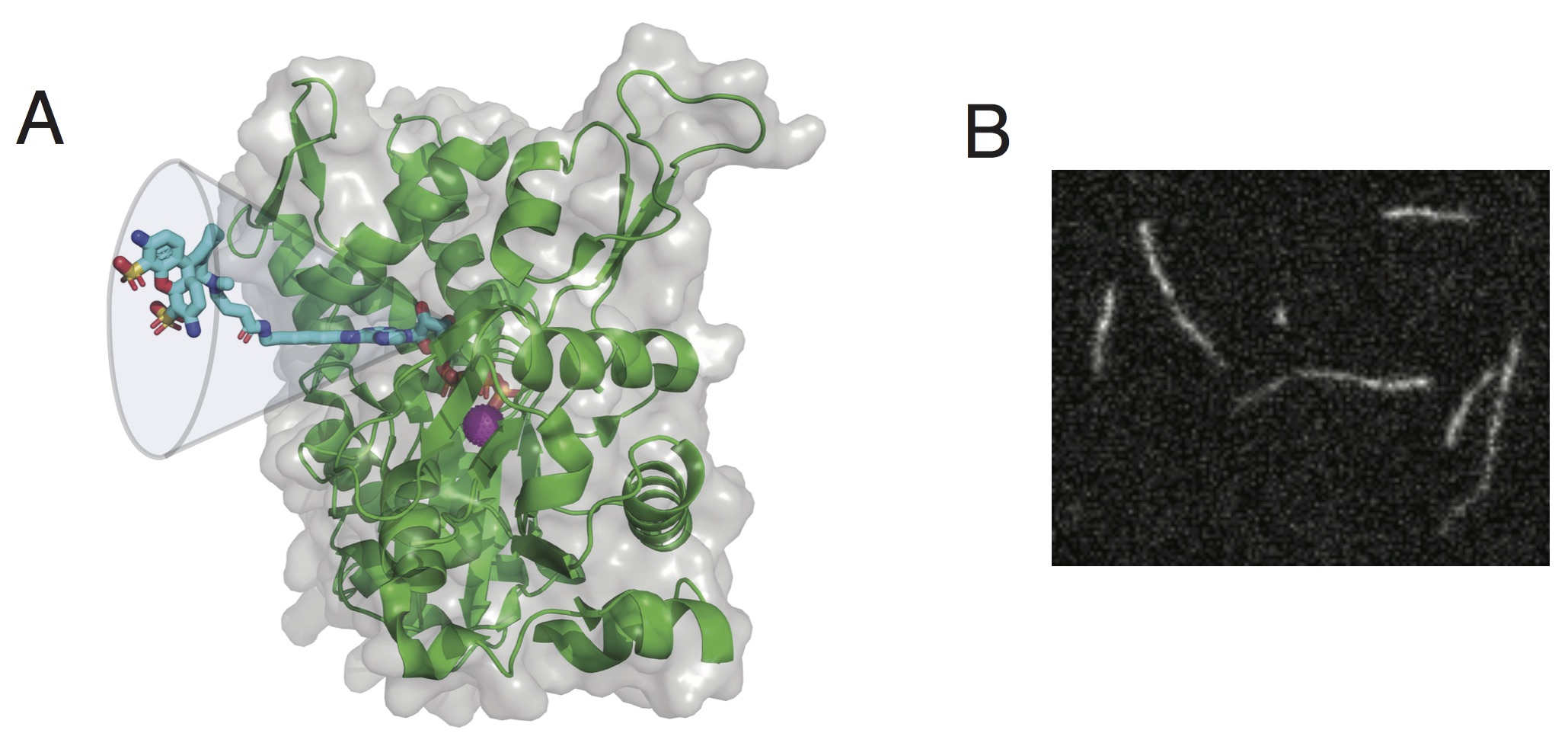
(B) Fluorescence image of a single actin filament labeled with ATP-ATTO-488.
To know more:
A functional family of fluorescent nucleotide analogues to investigate actin dynamics and energetics
Jessica Colombo, Adrien Antkowiak, Konstantin Kogan, Tommi Kotila, Jenna Elliott, Audrey Guillotin, Pekka Lappalainen & Alphée Michelot
Nature Communications volume 12, Article number: 548 (2021)
Contact
Alphée Michelot – alphee.michelot@univ-amu.fr