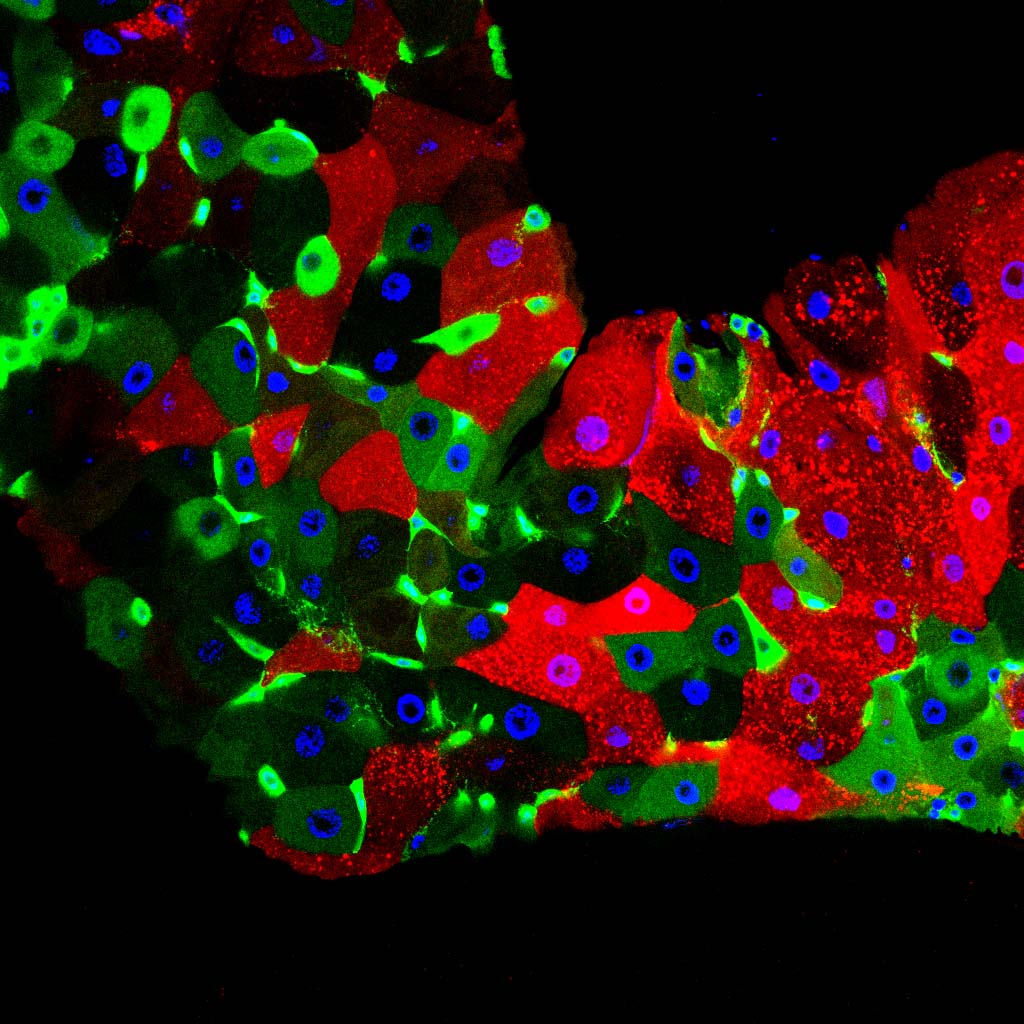
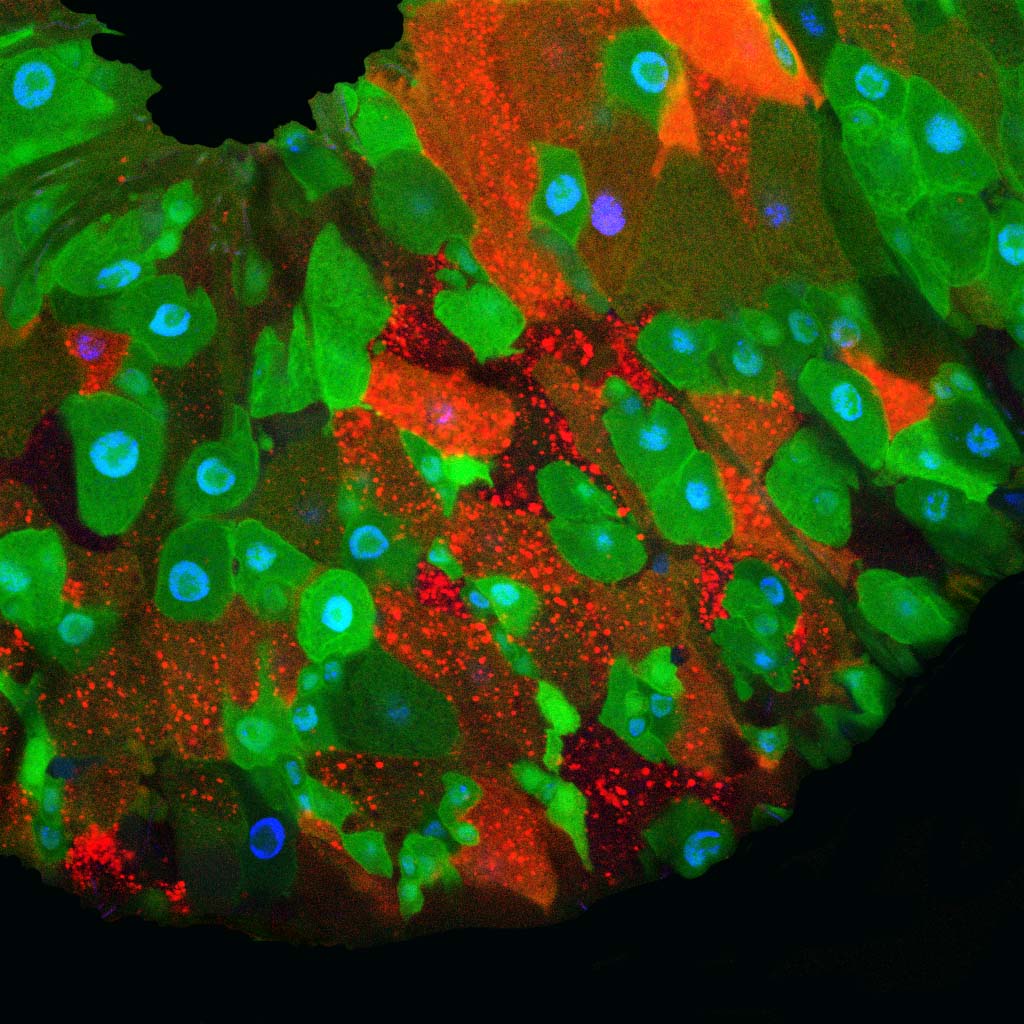
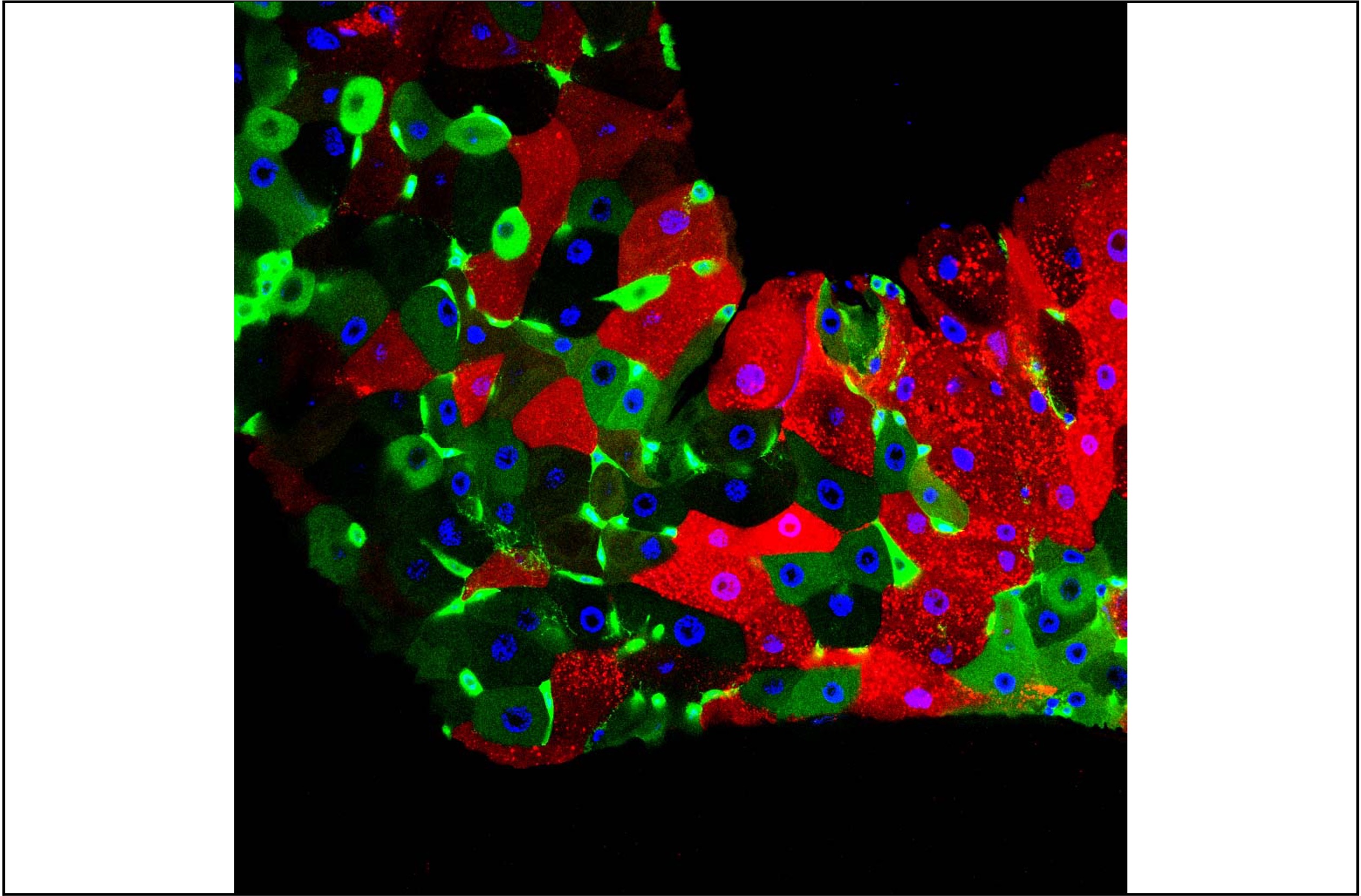
Bacteria that colonize eukaryotic gut have profound influences on the physiology of their host. In Drosophila, many of these effects are mediated by adipocytes that combine immune and metabolic functions. We found that enteric infection with some bacteria species triggers the activation of the lipogenic protein SREBP (Sterol Regulatory Element Binding Protein) in surrounding enterocytes but also in remote fat body cells and in ovaries, an effect that requires insulin signaling.
Our work demonstrates that by activating the NF-kB pathway, the cell wall peptidoglycan produced by the same gut bacteria remotely, and cell-autonomously, represses SREBP activation in adipocytes. By reducing the level of peptidoglycan, the gut born PGRP-LB amidase balances host immune and metabolic responses of the fat body to gut-associated bacteria. In the absence of such modulation, uncontrolled immune pathway activation prevents SREBP activation and lipid production by the fat body.