Maina Team publishes in Cell Death and Disease. Researchers have identified a new mechanism of genetic cooperation driving Hepatocellular Carcinoma.
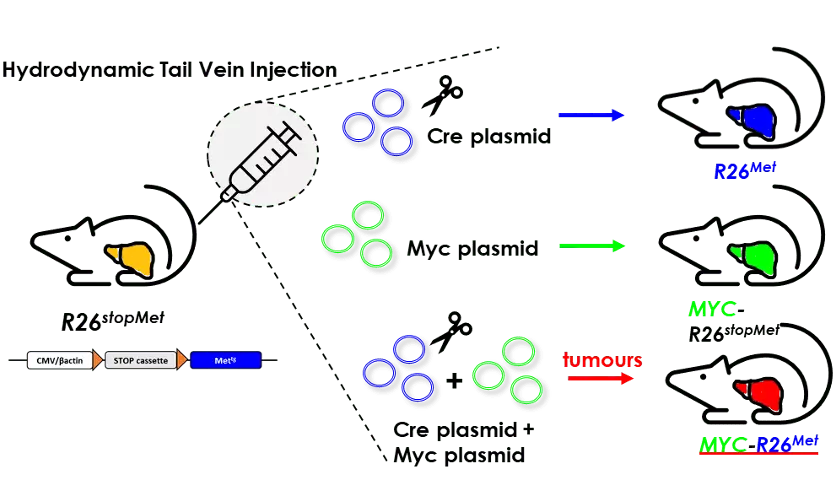
In a collaborative setting with the labs of Dr. K. Wangensteen (USA), Dr. G. Barrera and Dr. S. Pizzimenti (Italy), we have applied a powerful approach, named Hydrodynamic Tail Vein Injection, to transfect hepatocytes in vivo in the context of a mouse model with increased RTK levels. We functionally documented the cooperativity between MYC and MET in liver tumorigenesis, and how combined targeting of MYC and MET converts a partial cytostatic effect, triggered by individual blockage, into a cytotoxic effect. These findings may be particularly relevant for a subgroup of HCC patients characterised by high levels of MYC and MET, which we have identified in distinct cohorts.
This work has been commented by Fondation ARC: MYC et MET, « Bonnie and Clyde » hépatiques…
MYC and MET cooperatively drive hepatocellular carcinoma with distinct molecular traits and vulnerabilities
Summary
Enhanced activation of the transcription factor MYC and of the receptor tyrosine kinase MET are among events frequently occurring in hepatocellular carcinoma (HCC). Both genes individually act as drivers of liver cancer initiation and progression. However, their concomitant alteration in HCC has not been explored, nor functionally documented. Here, we analysed databases of five independent human HCC cohorts and found a subset of patients with high levels of MYC and MET (MYChigh/METhigh) characterised by poor prognosis. This clinical observation drove us to explore functionality of MYC and MET co-occurrence in vivo, combining hydrodynamic tail vein injection for MYC expression in the R26stopMet genetic setting, in which wild-type MET levels is enhanced following genetic deletion of a stop cassette. Results showed that increased MYC and MET expression in hepatocytes is sufficient to induce liver tumorigenesis even in the absence of pre-existing injuries associated with a chronic disease state. Intriguingly, ectopic MYC in MET tumours increases expression of the Mki67 proliferation marker, and switches them into loss of Afp, Spp1, Gpc3, Epcam accompanied by increased in Hgma1, Vim, and Hep-Par1 levels. We additionally found a switch in the expression of specific immune checkpoints, with an increase in the Ctla-4 and Lag3 lymphocyte co-inhibitory responses, and in the Icosl co-stimulatory responses of tumour cells. We provide in vitro evidence on the vulnerability of some human HCC cell lines to combined MYC and MET targeting, which are otherwise resistant to single inhibition. Mechanistically, combined blockage of MYC and MET converts a partial cytostatic effect, triggered by individual blockage of MYC or MET, into a cytotoxic effect. Together, these findings highlight a subgroup of HCC characterised by MYChigh/METhigh, and document functional cooperativity between MYC and MET in liver tumorigenesis. Thus, the MYC-R26Met model is a relevant setting for HCC biology, patient classification and treatment.
To know more
Celia Sequera1,#, Margherita Grattarola1,2,#, Agnes Holczbauer3, Rosanna Dono1, Stefania Pizzimenti2, Giuseppina Barrera2, Kirk Wangensteen3,*, and Flavio Maina1,*
1 Aix-Marseille Univ, CNRS, Developmental Biology Institute of Marseille (IBDM), Turing Center for Living Systems, Parc Scientifique de Luminy, Marseille (France).
2 Department of Clinical and Biological Science, University of Turin, 10125 Turin (Italy).
3 Division of Gastroenterology, Department of Medicine, Mayo Clinic, Rochester, (USA).
# These authors contributed equally to this work.
Cell Death and Disease 2022 Nov 24;13(11):994. doi: 10.1038/s41419-022-05411-6.
Contact
Figure legend
Concomitant upregulation of MYC and MET in a subset of hepatocytes triggers tumorigenesis in mice. The scheme illustrates the protocol of Hydrodynamic Tail Vein Injection (HTVI) used in R26stopMet mice with Cre, Myc, or Cre+Myc plasmids.