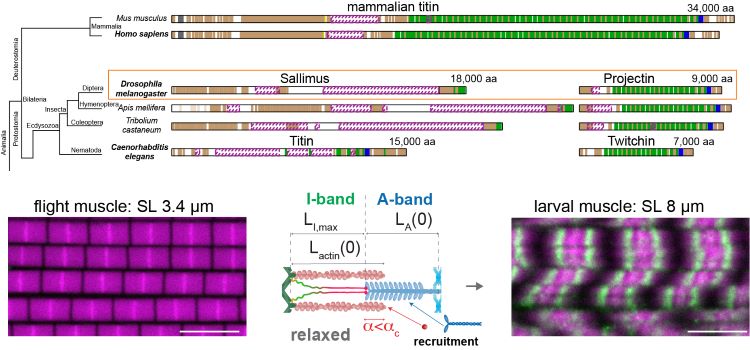
Figure: Drosophila muscle diversity is achieved by a split titin in insects. Mammals contain one large titin protein, while insects and nematodes split its function in two. A short version of the I-band variant Sallimus builds short flight muscle sarcomeres, whereas long Sallimus (green) makes long larval sarcomeres. These different sarcomere dimensions are achieved by differential recruitment of actin and myosin subunits.
Insects display a remarkable breadth of behaviours. They fly, with wing beat frequencies up to 1000 oscillations per second, they walk, with coordinated steps of their 6 legs, or they can cut leaves, requiring maximum force production with their mandibles. All these behaviours are powered by muscles housing a common contractile unit called a sarcomere. How can sarcomeres contract in such fundamentally different ways?
An interdisciplinary team with the Schnorrer and Habermann groups at IBDM, the Rupprecht group at CPT (all three in Marseille), together with the von Philipsborn group in Fribourg and the Görlich group in Göttingen now provide insight into how insects manage to tune the actin and myosin filaments in the sarcomere, as well as their overlap in the sarcomere, in a muscle type-specific way.
It was known that sarcomere dimensions in human muscle and heart are determined by the gigantic ruler protein titin. Paul De Boissier, PhD student in the Habermann group, constructed an evolutionary tree of titin and found that all insects and nematodes have two titin proteins, one spanning the sarcomere I-band, the other located on the A-band. Hence, a strict ruler model controlling the sarcomere dimensions, as found in human sarcomeres, cannot apply in insects.
Vincent Loreau and Wouter Koolhaas, PhD students in the Schnorrer group, discovered that the length of the elastic domain in the Drosophila I-band titin, called Sallimus, determines not only how long the actin filament containing I-band is, but surprisingly also how long the myosin filament containing A-band is: CRISPR-based shortening or lengthening of Sallimus resulted in shorter or longer actin and myosin filaments. The precise length of Sallimus was determined by specific nanobodies binding to its ends, developed by the Görlich group in Göttingen. Thus, longer Sallimus makes actin and myosin filaments longer. Sabina Avosani in the v. Philipsborn group determined the flight frequency of the long Sallimus flies: interestingly, they fly at lower flight frequency.
A second way to make Sallimus longer is to pull with higher force. Vincent Loreau developed a force sensor in Sallimus and showed that long larval I-band sarcomeres display high Sallimus forces, while short I-band flight muscles display low forces. Thus, a combination of Sallimus isoforms and forces determine the sarcomere dimensions and hence its biomechanical properties.
These biological data inspired Jean-Francois Rupprecht to develop a mathematical model that explains how I-band and A-band length scale together in a muscle type-specific way: both filament types grow until they reach a sufficient overlap to effectively contract. The contractions then block further length increase. This model was tested by Vincent Loreau and Eunice Chan experimentally by increasing or decreasing the active muscle contractions during muscle development. Decreasing the contractions indeed leads to longer sarcomeres with longer actin-myosin overlap, while increasing contractions results in shorter sarcomeres.
Together, these results explain how insects and possibly also nematodes can tune their sarcomere dimensions using a biomechanical feedback mechanism to achieve the observed sarcomere specialisations that allow a fast flight a coordinated walk in the same animal.
Loreau V, Koolhaas WH, Chan EH, De Boissier P, Brouilly N, Avosani S, Sane A, Pitaval C, Reiter S, Luis NM, Mangeol P, von Philipsborn AC, Rupprecht JF, Görlich D, Habermann BH, Schnorrer F. Titin-dependent biomechanical feedback tailors sarcomeres to specialized muscle functions in insects. Sci Adv. 2025 May 9;11(19):eads8716.