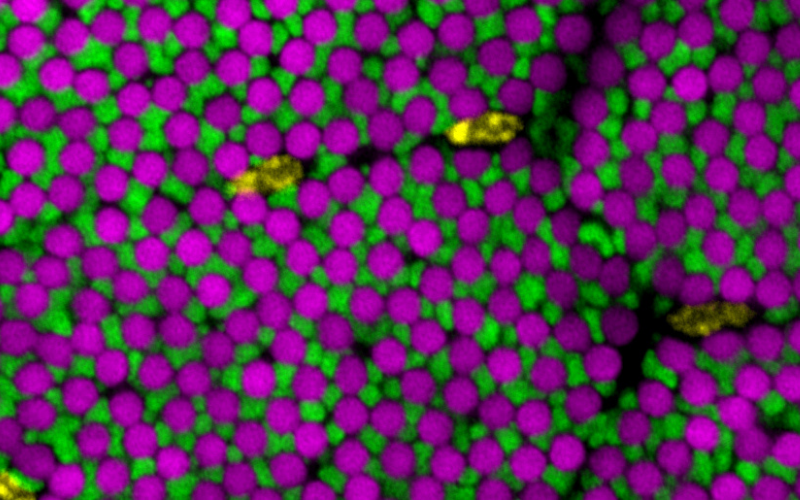
Figure: Drosophila flight muscle internal architecture in a cross-section: myofibrils (magenta) are tightly packed in proximity to mitochondria (green) present in distinct bundles, with nuclei (yellow) located in between the bundles.
Muscles are built of large, force-producing cells. The force is generated by contractile filaments called myofibrils, while the required energy is provided by adjacent mitochondria. Mitochondria and myofibrils occupy most of the muscle’s volume; interestingly their relative distribution is highly organised and specific to the respective muscle type, optimised for maximum power or enduring contractions. How this intricate organization is set up during
muscle development has been unclear. Using live imaging and genetic tricks, Jerome Avellaneda and colleagues at IBDM in Marseille now identified microtubules as the key architect, bringing mitochondria in close proximity to myofibrils: microtubules tightly bundle around the assembling myofibrils and tell them their correct orientation in the large developing Drosophila flight muscles. Then, the microtubule motor kinesin, transports mitochondria along the same microtubules and thus brings them close to each myofibril, insulating one myofibril from its neighbour (see image). This way, energy production site come in maximum proximity to the energy consuming muscle motors, ensuring long-lasting insect flight. In collaboration with the group of Edgar Gomes at IMM in Lisbon, Jerome found that the key role of microtubules in coordinating myofibril with mitochondria morphogenesis is also conserved in cultured mouse myofibers. This work is now published in Developmental Cell.
Avellaneda J, Candeias D, da Rosa Soares A, Gomes ER, Luis NM, Schnorrer F. Microtubules coordinate mitochondria transport with myofibril morphogenesis during muscle development. Dev Cell. 2025 Jul 15:S1534-5807(25)00411-3.