Imagerie optique
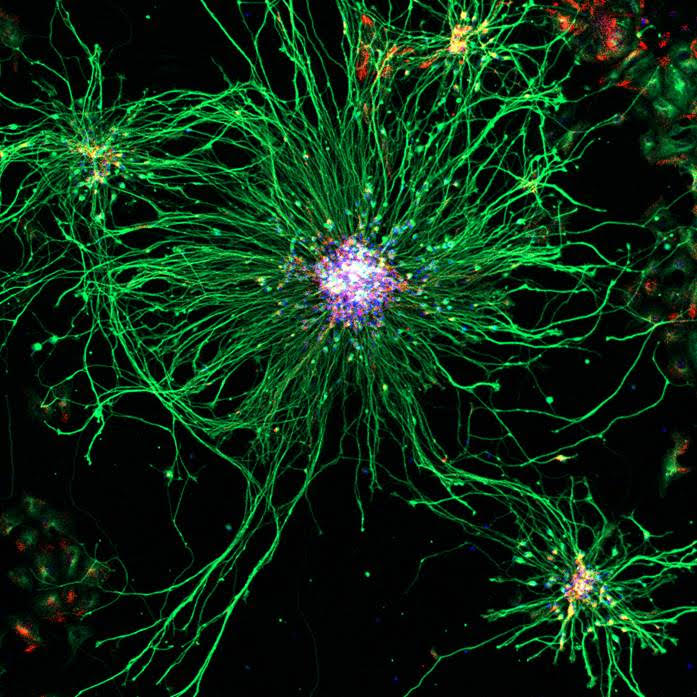
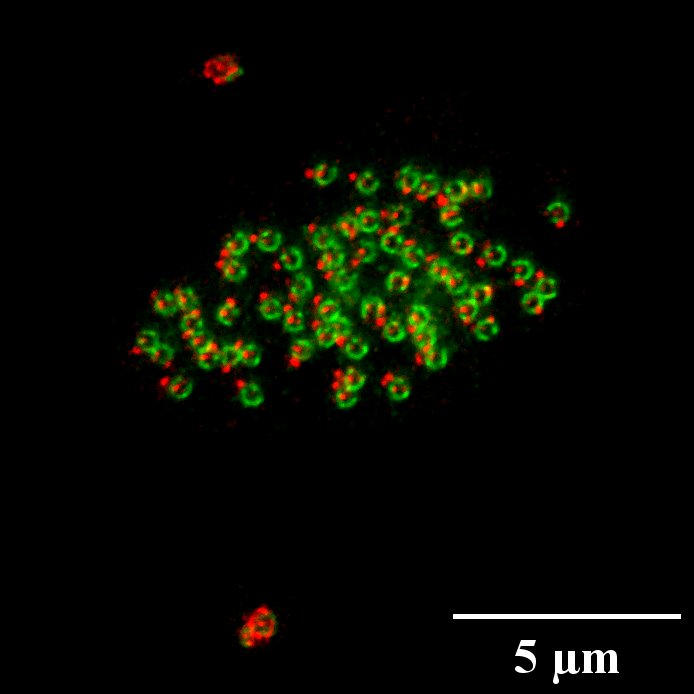
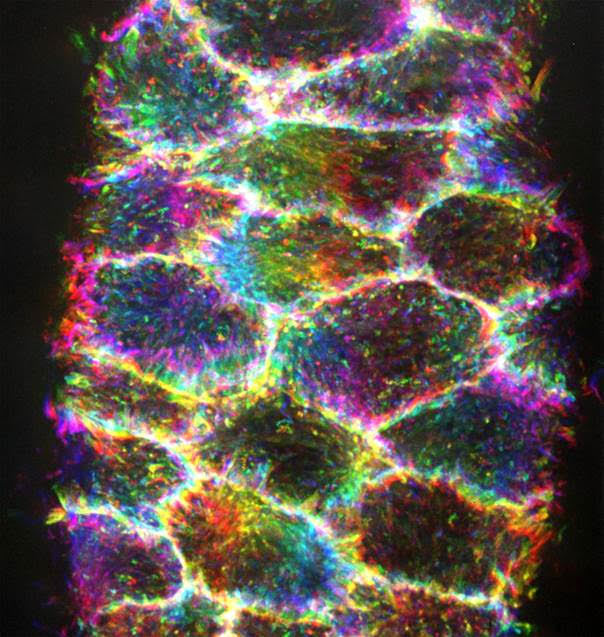
Microscopie optique pour l'imagerie biologique, de l'échelle subcellulaire à celle de l'organisme entier
Le département de microscopie optique du centre d’imagerie PiCsL-IBDM fournit à la communauté scientifique locale, nationale et internationale une expertise et des systèmes d’imagerie optique pour l’imagerie à multi-échelle, des cellules aux petits organismes entiers. Notre offre de services comprend des conseils techniques, des formations et des conceptions expérimentales
La tarification de la réservation des microscopes, de notre offre de services et de notre offre de formation est basée sur des tarifs validés par le CNRS.
Contactez l'équipe
Avez-vous besoin de nos services ?
N'hésitez pas à nous contacter
Équipements
Équipements
Techniques standard de microscopie avec contraste de fluorescence limité par diffraction
Publications
Nos dernières publications
Actualités
de l'équipe
Articles
Offres d'emploi
Articles
No Posts found..
Offres d'emploi
No jobs opportunities found..
Membres de l'équipe
Personnel technique à votre service
Ingénieur/Technicien
Ingénieur/Technicien
Ingénieur/Technicien
Ingénieur/Technicien