Membres de l'équipe
Hanyu Wang
Dynamique musculaire
Nous étudions la mécanobiologie du muscle. Nous nous intéressons à la façon dont les muscles fonctionnels sont fabriqués pendant le développement et comment ils restent fonctionnels tout au long de la vie d'un animal.
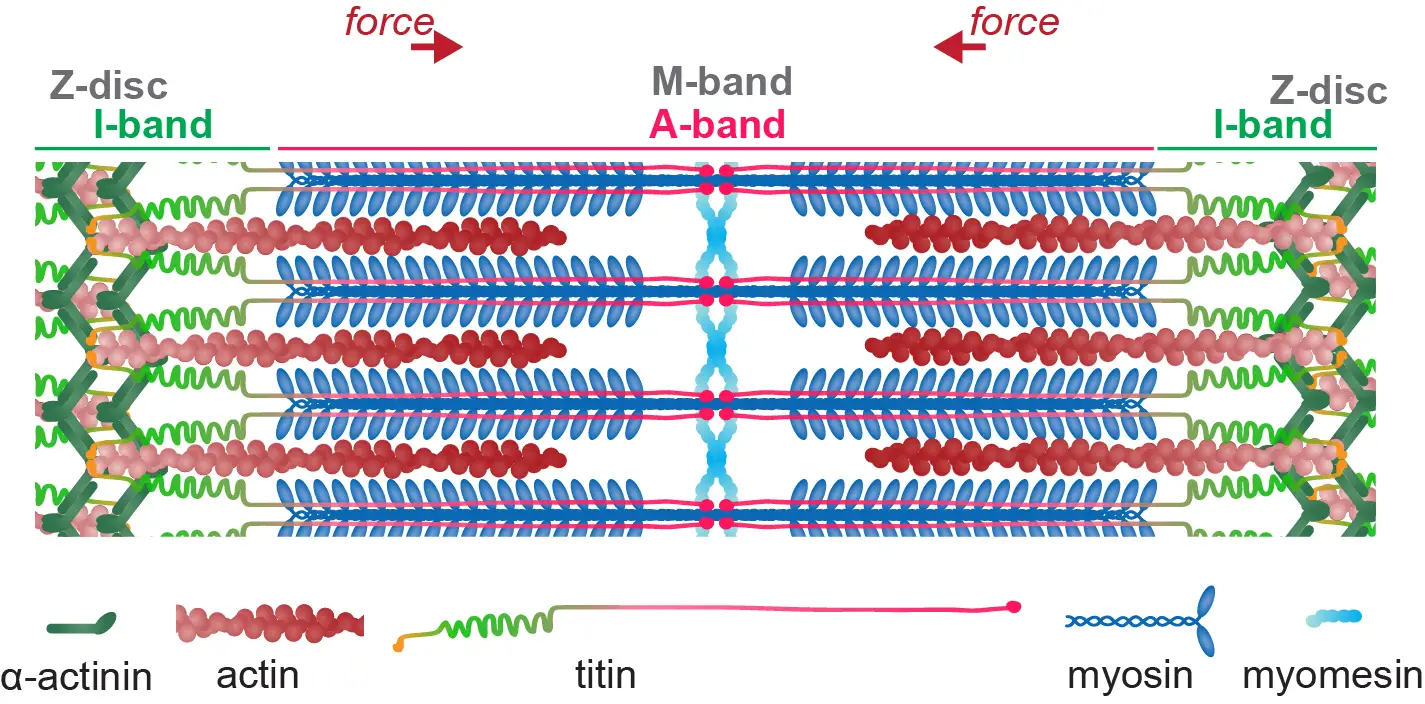
La force de notre groupe réside dans sa combinaison unique de génétique systématique et de biologie cellulaire in vivo en direct, avec la mécano- et la biologie structurale. Nous utilisons des modèles in vivo de drosophile et in vitro d’iPSC humains pour étudier les mécanismes d’assemblage par les muscles de leurs sarcomères contractiles et la manière dont ces sarcomères sont entretenus chez l’animal vivant pour rester fonctionnels tout au long de la vie. Les sarcomères sont parmi les plus grands assemblages de protéines chez les animaux : ils produisent des forces élevées et se lient mécaniquement au squelette pour alimenter le mouvement animal. Donc, les sarcomères sont un fantastique terrain de jeu pour comprendre les principes de base de la biologie:
Comment des milliers de grosses protéines s’assemblent-elles pour construire un sarcomère pseudo-cristallin d’une taille de l’ordre du micromètre ?
Comment des milliers de sarcomères s’auto-assemblent-ils en chaînes qui se connectent mécaniquement à travers des fibres musculaires de centimètres de long ?
Comment le développement et l’entretien des sarcomères sont-ils coordonnés avec les autres exigences physiologiques des cellules musculaires, notamment la biogénèse des mitochondries, la formation des tubules T et le retournement protéasomique des protéines endommagées tout au long de la vie ?
La réponse à ces questions nous permettra de mieux comprendre comment les muscles sont effectivement construits au cours du développement, comment ils s’adaptent à leurs différents besoins physiologiques (voir muscles cardiaques vs muscles squelettiques) et comment ils restent fonctionnels tout au long de notre vie.
Publications
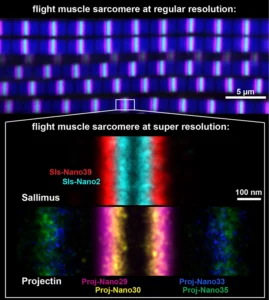
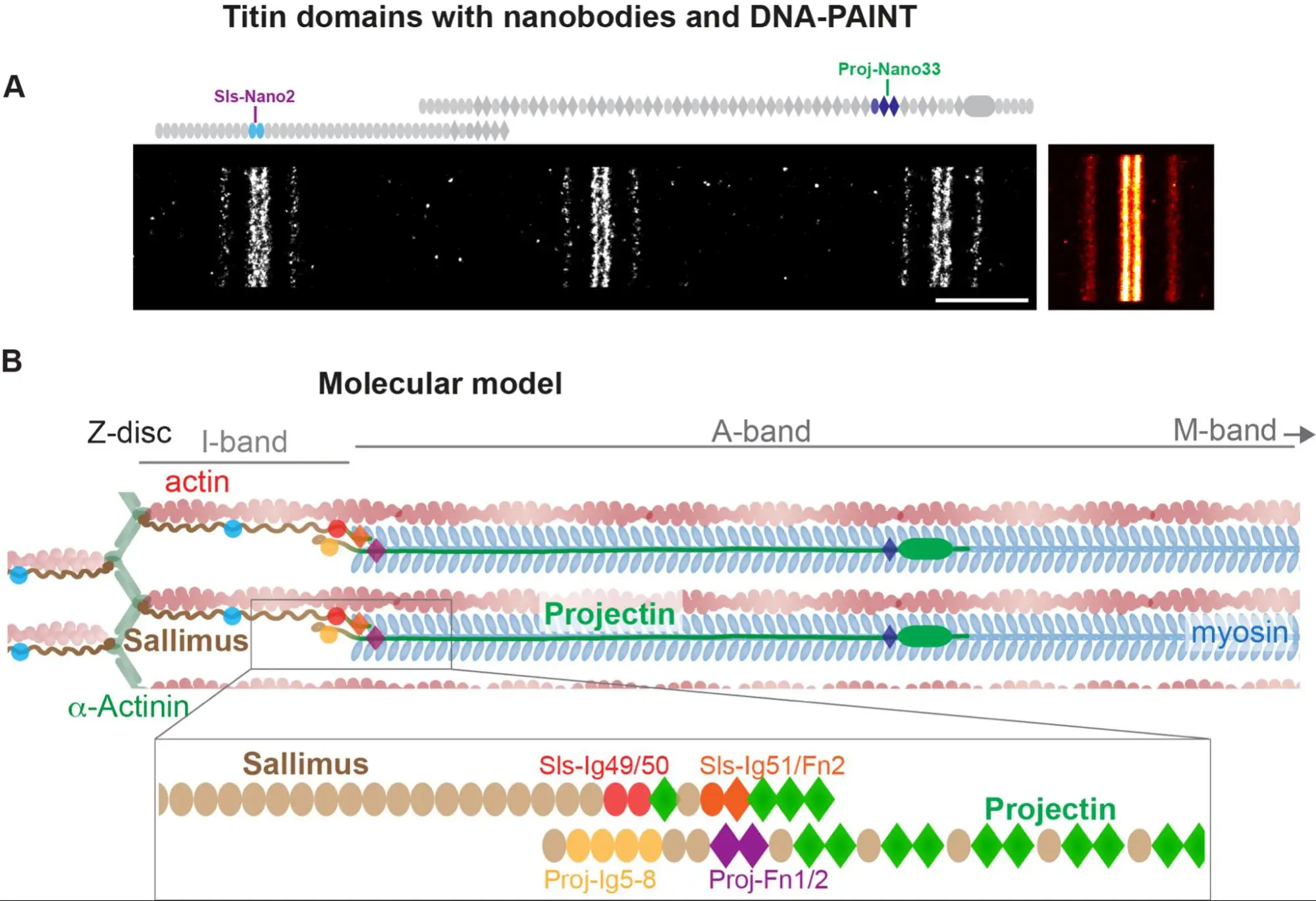
Nanobodies combined with DNA-PAINT super-resolution reveal a staggered titin nanoarchitecture in flight muscles
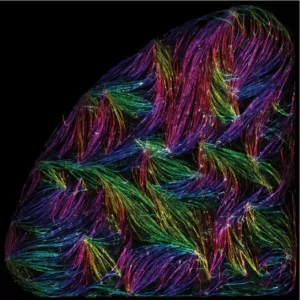
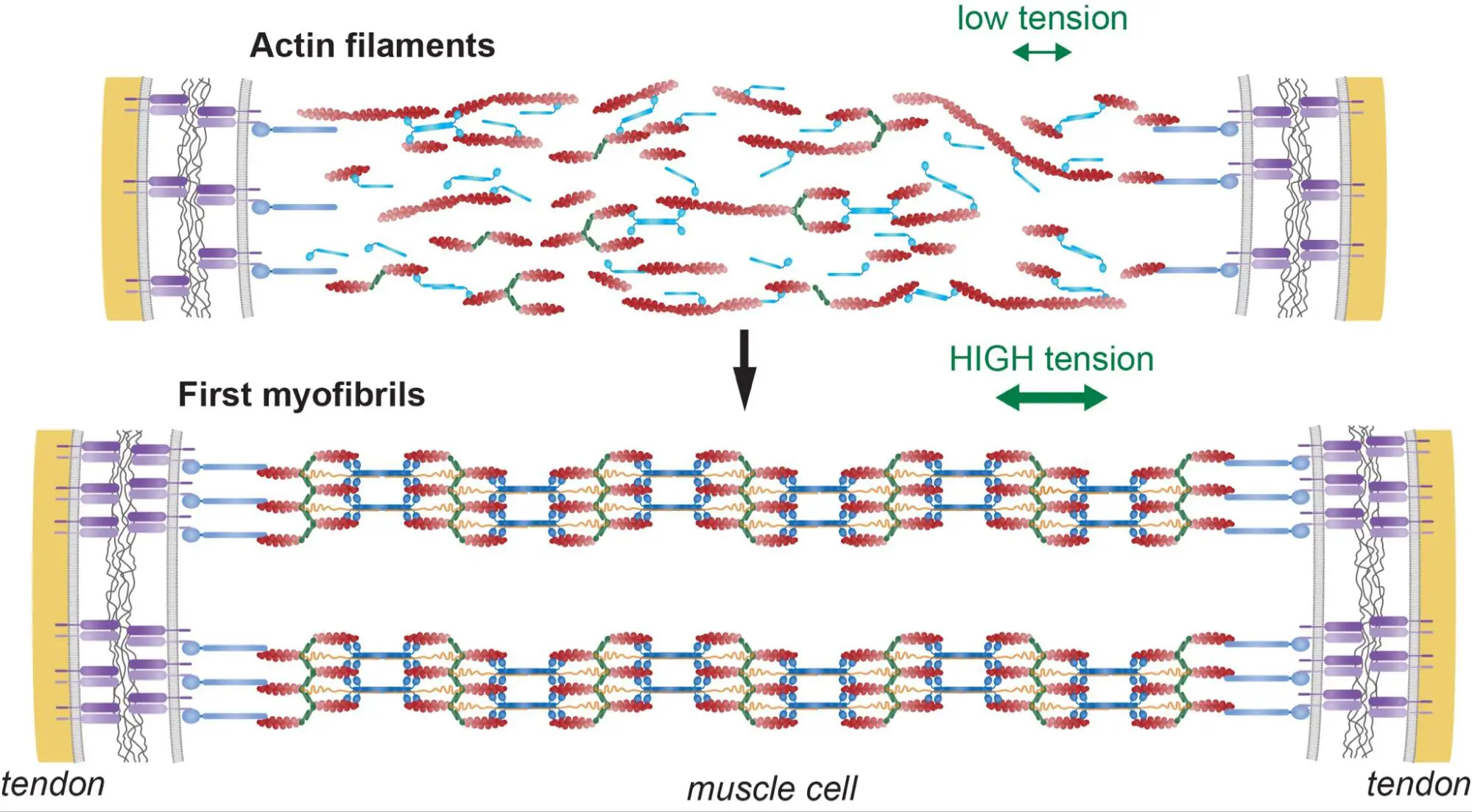
Tension-driven multi-scale self-organisation in human iPSC-derived muscle fibers
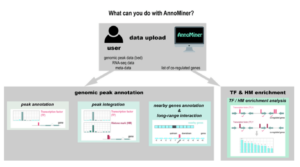
AnnoMiner is a new web-tool to integrate epigenetics, transcription factor occupancy and transcriptomics data to predict transcriptional regulators
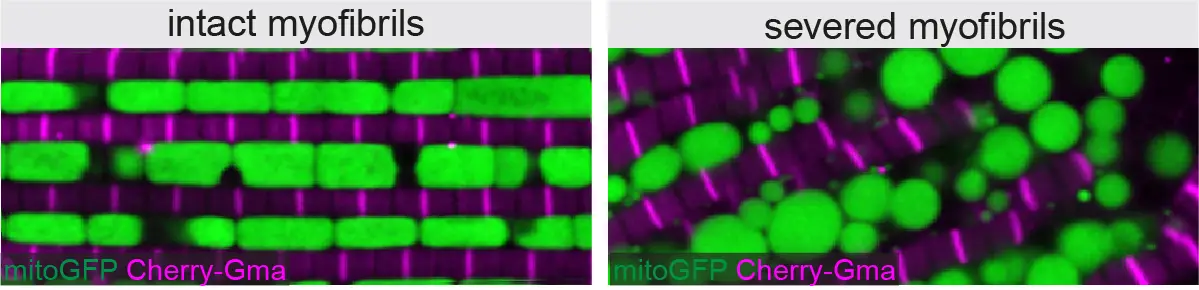
Myofibril and mitochondria morphogenesis are coordinated by a mechanical feedback mechanism in muscle
The Hippo pathway controls myofibril assembly and muscle fiber growth by regulating sarcomeric gene expression
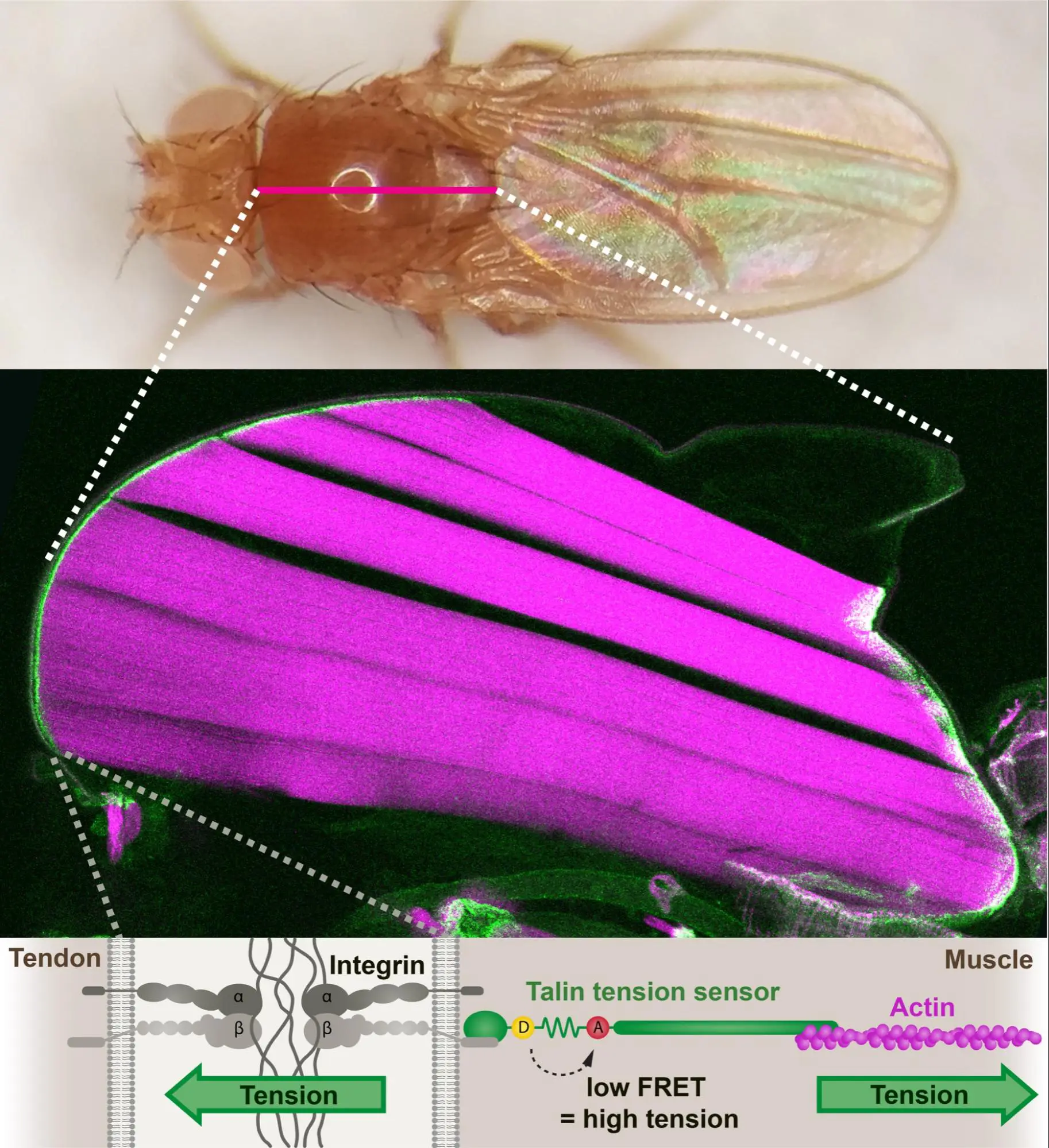
A small proportion of Talin molecules transmit forces at developing muscle attachments in vivo
A nanobody toolbox to investigate localisation and dynamics of Drosophila titins and other key sarcomeric proteins
Nanobodies combined with DNA-PAINT super-resolution reveal a staggered titin nanoarchitecture in flight muscles
Tension-driven multi-scale self-organisation in human iPSC-derived muscle fibers
Tagging Drosophila Proteins with Genetically Encoded Fluorophores
Fly Cell Atlas: A single-nucleus transcriptomic atlas of the adult fruit fly
Mechanobiology of muscle and myofibril morphogenesis
AnnoMiner is a new web-tool to integrate epigenetics, transcription factor occupancy and transcriptomics data to predict transcriptional regulators
Mechanobiology: Forging a strong matrix at tendons
Myofibril and mitochondria morphogenesis are coordinated by a mechanical feedback mechanism in muscle
The Hippo pathway controls myofibril assembly and muscle fiber growth by regulating sarcomeric gene expression
A small proportion of Talin molecules transmit forces at developing muscle attachments in vivo
A transcriptomics resource reveals a transcriptional transition during ordered sarcomere morphogenesis in flight muscle.
Ordering of myosin II filaments driven by mechanical forces: experiments and theory.
Polarization-resolved microscopy reveals a muscle myosin motor-independent mechanism of molecular actin ordering during sarcomere maturation.
In Vivo Imaging of Muscle-tendon Morphogenesis in Drosophila Pupae.
Gene Tagging Strategies To Assess Protein Expression, Localization, and Function in Drosophila.
AIDing-targeted protein degradation in Drosophila.
Mechanical tension and spontaneous muscle twitching precede the formation of cross-striated muscle in vivo.
A Guide to Genome-Wide In Vivo RNAi Applications in Drosophila.
A genome-wide resource for the analysis of protein localisation in Drosophila.
Slit cleavage is essential for producing an active, stable, non-diffusible short-range signal that guides muscle migration.
The RNA-binding protein Arrest (Bruno) regulates alternative splicing to enable myofibril maturation in Drosophila flight muscle.
Tension and force-resistant attachment are essential for myofibrillogenesis in Drosophila flight muscle
Ret rescues mitochondrial morphology and muscle degeneration of Drosophila Pink1 mutants
Transcriptional regulation and alternative splicing cooperate in muscle fiber-type specification in flies and mammals
A simple protocol to efficiently engineer the Drosophila genome by TALENs
Spalt mediates an evolutionary conserved switch to fibrillar muscle fate in insects
The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures.
Systematic genetic analysis of muscle morphogenesis and function in Drosophila
Three-dimensional reconstruction and segmentation of intact Drosophila by ultramicroscopy.
In vivo RNAi rescue in Drosophila melanogaster with genomic transgenes from Drosophila pseudoobscura
High-resolution, high-throughput SNP mapping in Drosophila melanogaster
Ultramicroscopy: 3D reconstruction of large microscopical specimens
Positional cloning by fast-track SNP-mapping in Drosophila melanogaster
A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila.
The transmembrane protein Kon-tiki couples to Dgrip to mediate myotube targeting in Drosophila
The gammaTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline
RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation
Muscle building; mechanisms of myotube guidance and attachment site selection
Gamma-tubulin37C and gamma-tubulin ring complex protein 75 are essential for bicoid RNA localization during drosophila oogenesis
The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes.
Oligomerisation of Tube and Pelle leads to nuclear localisation of dorsal
The cellular localization of the murine serine/arginine-rich protein kinase CLK2 is regulated by serine 141 autophosphorylation
Actualités
Fais ton stage à l’IBDM !
Tu es à la recherche de ton stage de Master ? L’IBDM te semble être le bon endroit pour le faire ? Découvre nos offres dès maintenant.
Félicitation à Robert Kelly, Frank Schnorrer, Cédric Maurange, Bianca Habermann et Delphine Delacour !
De post-doctorante (bourse AFM-Téléthon) à chercheuse au CNRS : Qiyan Mao fait progresser la recherche sur les muscles humains grâce à des approches en mécanique moléculaire et tissulaire.
L’IBDM inspire les jeunes esprits : en impliquant les enfants des écoles primaires dans la lutte contre le cancer pédiatrique (“Contre le cancer, j’apporte ma pierre”) et en interagissant avec les lycéens grâce à des expériences immersives (DECLICS).
Séminaire Interne par Nuno Luis
Une approche interdisciplinaire pour explorer et cibler la dialectique cancer-cellules immunitaires : d’un modèle de souris TNBC aux patients
Les sarcomères des drosophiles sondés à l’échelle nanométrique une molécule à la fois à l’aide de nanocorps.
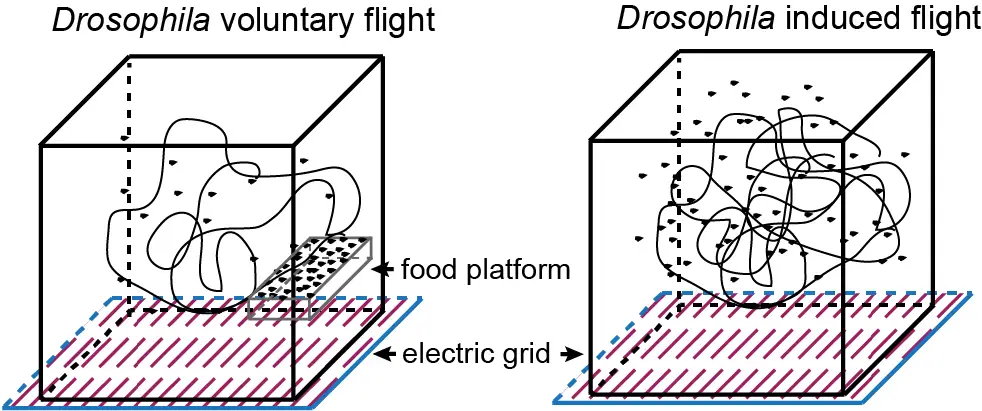
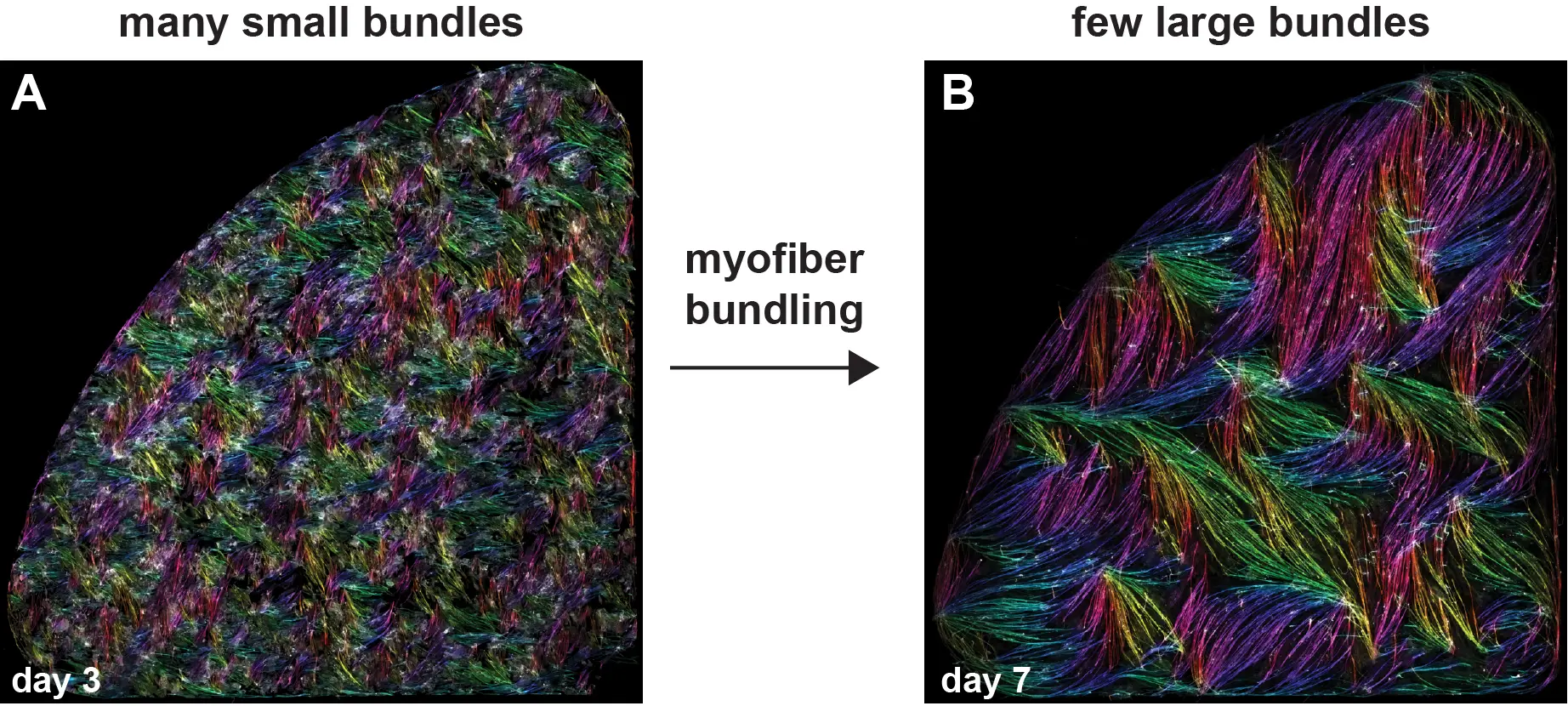
Les cellules musculaires s’auto-organisent en faisceaux de fibres in vitro, sans la présence de signaux externes !
Frank Schnorrer élu membre de l’EMBO
L’EMBO élit 67 nouveaux membres et membres associés. Ils rejoignent la communauté de plus de 1 900 scientifiques du vivant de premier plan en Europe et au-delà.
Nous présentons ‘AnnoMiner’, un nouvel outil convivial basé sur le web pour annoter et intégrer les données épigénétiques et de liaison des facteurs de transcription.
En combinant la génétique chez la drosophile avec l’imagerie de pointe et le “deep-learning”, des chercheurs de l’IBDM ont découvert que les mitochondries coordonnent leur formation avec le développement des myofibrilles pour correspondre au bon type musculaire.
L’équipe de Schnorrer et al. ont découvert qu’une voie de signalisation, appelée voie Hippo, contrôle la croissance musculaire pendant le développement des muscles du vol, chez la drosophile.
Le Conseil européen de la recherche (ERC) a attribué l’un des rares financements ERC Synergy à un consortium international de scientifiques, Frank Schnorrer, Stefan Raunser, Dirk Görlich et Mathias Gautel.
Investigating the mechanobiology of self-organisation in human iPSC-derived skeletal muscles
The successful completion of the project will allow you to answer how mechanical tension is generated inside human myofibers by integrating intracellular molecular forces and extracellular mechanical environment.
Investigating the mechanobiology of self-organisation in human iPSC-derived skeletal muscles
Annonce en anglais Project title: Investigating the mechanobiology of self-organisation in human iPSC-derived skeletal muscles Type of rotation: M1 (2 months) or M2 (6 months)